Publications
Highlights
(For a full list see below)
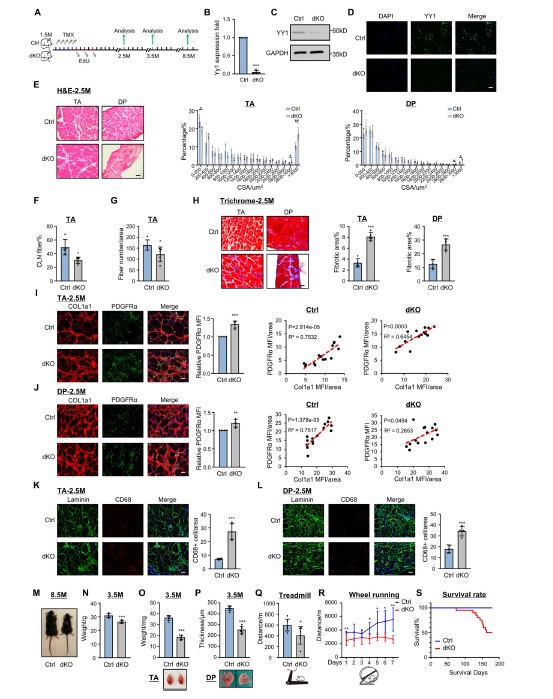
Adult skeletal muscle stem cells (MuSCs) are indispensable for muscle regeneration and tightly regulated by macrophages (MPs) and fibroadipogenic progenitors (FAPs) in their niche. Deregulated MuSC/MP/FAP interactions and the ensuing inflammation and fibrosis are hallmarks of dystrophicmuscle. Here we demonstrate intrinsic deletion of transcription factor Yin Yang 1 (YY1) in MuSCs exacerbates dystrophic pathologies by altering composition and heterogeneity of MPs and FAPs. Further analysis reveals YY1 loss induces expression of immune genes in MuSCs, including C-C motif chemokine ligand 5 (Ccl5). Augmented CCL5 secretion promotes MP recruitment via CCL5/C-C chemokine receptor 5 (CCR5) crosstalk, which subsequently hinders FAP clearance through elevated Transforming growth factor-β1 (TGFβ1). Maraviroc-mediated pharmacological blockade of the CCL5/CCR5 axis effectively mitigates muscle dystrophy and improves muscle performance. Lastly, we demonstrate YY1 represses Ccl5 transcription by binding to its enhancer thus facilitating promoter-enhancer looping. Altogether, our study demonstrates the critical role of MuSCs in actively shaping their niche and provides novel insight into the therapeutic intervention of muscle dystrophy.
Yang Li*, Chuhan Li, Qiang Sun, Xingyuan Liu, Fengyuan Chen, Yeelo Cheung, Yu Zhao, Ting Xie, Bénédicte Chazaud, HaoSun, Huating Wang
Nature Communications, 2025, DOI: 10.1038/s41467-025-56474-w
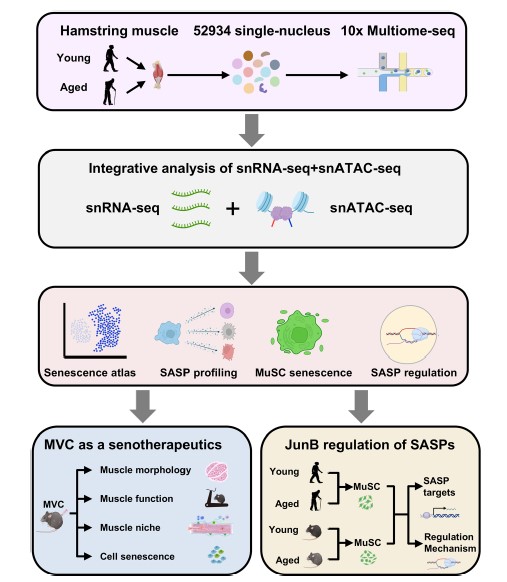
Cellular senescence is a hallmark of organismal aging but how it drives aging in human tissues is not fully understood. Here we leverage single nucleus multiomics to profile senescence inmononucleated cells of human skeletalmuscle and provide the first senescence atlas. We demonstrate the intra- and interpopulational transcriptomic and epigenomic heterogeneity and dynamics of cellular senescence. We also identify commonalities and variations in senescence-associated secretory phenotypes (SASPs) among the cells and elucidate SASP mediated cellular interactions and niche deregulation. Furthermore, we identify targetable SASPs and demonstrate the possibility of using Maraviroc as a pharmacological senotherapeutic for treating ageassociated sarcopenia. Lastly, we define transcription factors that govern senescence state and SASP induction in aging muscle and elucidate the key function and mechanism of JUNB in SASP activation. Altogether, our findings demonstrate the prevalence and function of cellular senescence in skeletal muscle and identify a novel pharmacological intervention for sarcopenia.
Yang Li*, Chuhan Li, Qin Zhou, Xingyuan Liu, Yulong Qiao, Ting Xie, Hao Sun, Michael Tim-Yun Ong, Huating Wang
Nature Communications, 2025, DOI: 10.1038/s41467-025-61403-y
Full List
2025
- Jinjin Kang, Yaxin Zeng, Nianle Li, Liangqiang He, Huating Wang, Kun Sun & Yu Zhao; Protocol for investigating chromatin architecture in adult skeletal muscle stem cells using high-throughput chromosome conformation capture STAR Protoc., 2025, DOI: 10.1016/j.xpro.2025.103962
- Lifang Han, Yudan Qiu, Liangqiang He, Chuan Gao, Nan Zhou, Xinrong Fu, Jingyan Xu, Lirong Lin, Huating Wang, Shyam Biswal & Zhenguo Wu; Non-canonical roles of Keap1/Nrf2 in regulating quiescence and early activation in adult muscle stem cells Nature Communications, 2025, DOI: 10.1038/s41467-025-65506-4
- Nuermila Yiliyaer, Xiaocheng Li, Tianyu Guo, Haiying Zhou, Lihai Gong, Luowei Yuan, Yang Fu, Yulong Qiao, Ying Lam Lui, Nuo Chen, Pengfei Lin, Hoi Hung Cheung, Ho Ko, Linyan Meng, Xiao Chen, Yong Lei, Kin Ming Kwan, Huating Wang, Shen Gu; Pathogenicity of Mediator Complex Subunit 27 (MED27) in a Neurodevelopmental Disorder with Cerebellar Atrophy. Advanced Science, 2025, DOI: 10.1002/advs.202505535
-
The X-Age Project to construct a Chinese aging clock Nature Aging; 2025, DOI: 10.1038/s43587-025-00935-w
- Jiaming Li, Mengmeng Jiang, Qiaoran Wang, Zikai Zheng, Jianghua Shen
- Jingyi Li, Muzhao Xiong, Yandong Zheng, Xiaoyong Lu, Yusheng Cai
- Yanling Fan, Lingling Geng, Qianzhao Ji, Qianqian Peng, Shuhui Sun
- Yuanyuan Wang, Zijuan Xin, Kaowen Yan, Yuanhan Yang, Jun Yu
- Haoteng Yan, Ding Ai, Yongping Bai, Yan Bi, Xiu-Wu Bian
- Pengcheng Bu, Jian-Ping Cai, Chun-Mei Cao, Feng Cao, Zhongwei Cao
- Renjie Chai, Piu Chan, Chang Chen, Cheng-Shui Chen, Chunying Chen
- Di Chen, Hou-Zao Chen, Lin Chen, Quan Chen, Xiao Chen, Xiaochun Chen
- Yu Chen, Zi-Jiang Chen, Weimin Ci, Zhe Dai, Qiurong Ding, Birong Dong
- Jiahong Dong, Jian-Gao Fan, Shiqing Feng, Xin Feng, Yun Feng
- Xiaobing Fu, Xiaolong Fu, Feng Gao, Jiangang Gao, Qiang Gao, Shaorong Gao
- Yonghao Gu, Youfei Guan, Feifan Guo, Jing-Dong J. Han, Haiping Hao
- Jihui Hao, Fuchu He, Jinhan He, Ming He, Mingguang He, Qiyang He
- Zhiying He, Zuhong He, Huashan Hong, Jiaxu Hong, Shengping Hou
- Cheng Hu, Ping Hu, Zhibin Hu, Canhua Huang, Jun Huang, Kai Huang
- Pengyu Huang, Xunming Ji, Yong Ji, Shunji Jia, Hong Jiang, Wenjian Jiang
- Lingjing Jin, Zi-Bing Jin, Shenghong Ju, Zhenyu Ju, Qing-Peng Kong, Wei Kong
- Wei-Jia Kong, Xiangqing Kong, Guanghua Lei, Geng-Lin Li, Ji Li, Jian Li
- Mengfeng Li, Rong Li, Wei Li, Wei Li, Xiao-Jun Li, Xin Li
- Qingfeng Liang, Zhen Liang, Haotian Lin, Baohua Liu, Cai-Yue Liu, Changsheng Liu
- Feng Liu, Jianfeng Liu, Jun-Ping Liu, Ke Liu, Lin Liu, Pingsheng Liu
- Qiang Liu, Qiang Liu, Tiemin Liu, Wenwen Liu, Xingguo Liu, Yajun Liu
- Yong Liu, Youhua Liu, Youshuo Liu, Zhili Liu, Xiao Long, Yao Lu
- Jian Luo, Xianghang Luo, Chunhong Ma, Shuai Ma, Xinran Ma, Jianhua Mao
- Zhiyong Mao, Shyh-Chang Ng, Guangjun Nie, Yuyu Niu, Yaojin Peng
- Jun Pu, Jieyu Qi, Li Qiang, Jie Qiao, Yingying Qin, Aijuan Qu
- Jing Qu, Jie Ren, Ruibao Ren, Xiong Z. Ruan, Anbing Shi, Haibo Shi
- Jie Shi, Kwok-Fai So, Moshi Song, Weihong Song, Zhou Songyang, Jiacan Su
- Aijun Sun, Liang Sun, Qiang Sun, Yi Eve Sun, Yu Sun, Peifu Tang
- Qi-Qun Tang, Yi Tang, Jun Tao, Ling Tao, Mei Tian, Xiao-Li Tian
- Ye Tian, Xiaolin Tong, Cong-Yi Wang, Haibo Wang, Hongmei Wang
- Huating Wang, Jianan Wang, Jianwei Wang, Jianwei Wang, Jiqiu Wang
- Liheng Wang, Lin Wang, Miao Wang, Qiang Wang, Si Wang, Sijia Wang
- Songlin Wang, Wengong Wang, Xiaoming Wang, Xiaoning Wang, Yan Wang
- Yan-Jiang Wang, Yuan Wang, Yunfang Wang, Zhenning Wang, Xiawei Wei
- Jianping Weng, Haitao Wu, Jihong Wu, Xiaohuan Xia, Yang Xia, Andy Peng Xiang
- Guozhi Xiao, Junjie Xiao, Yichuan Xiao, Zhi-Xiong Jim Xiao, Zhengwei Xie
- Wei Xiong, Aimin Xu, Hua Xu, Lingyan Xu, Ming Xu, Liying Yan, Jiayin Yang
- Jichun Yang, Liu Yang, Yun-Gui Yang, Ze Yang, Zhenglin Yang, Hongjie Yao
- Jing Ye, Chengqi Yi, Fan Yi, Honghua Yu, Yang Yu, Zhengrong Yu, Ti-Fei Yuan
- Jirong Yue, Rui Yue, Chen Zhang, Chunxiang Zhang, Cuntai Zhang, Feng Zhang
- Hongbo Zhang, Hongjia Zhang, Huijie Zhang, Jie Zhang, Jingjing Zhang, Licheng Zhang
- Lingqiang Zhang, Luoying Zhang, Qingjiong Zhang, Wei Zhang, Weiping J. Zhang, Xin Zhang
- Xuan Zhang, Yong Zhang, Yun-Wu Zhang, Zhanjun Zhang, Zhuohua Zhang, Bing Zhao
- Guoguang Zhao, Jiajun Zhao, Meng Zhao, Tongbiao Zhao, Jialin C. Zheng, Junke Zheng
- Zhuozhao Zheng, Huixia Zhou, Lili Zhou, Xiangtian Zhou, Yongsheng Zhou, Zhongjun Zhou
- Lan Zhu, Yizhun Zhu, Zhiming Zhu, Wenjuan Zhuang, Weiguo Zou, Weiqi Zhang, Gang Pei & Guang-Hui Liu
- Zeming Wu, Jing Qu, Weiqi Zhang, Aging Biomarker Consortium, Guang-Hui Liu Biomarkers of ageing of humans and non-human primates Nature Reviews Molecular Cell Biology; 2025, DOI: 10.1038/s41580-025-00883-8
- Xiaona Chen, Feng Yang, Suyang Zhang, Xiaofan Guo, Jieyu Zhao, Yulong Qiao, Liangqiang He, Yang Li, Qin Zhou, Michael Tim-Yun Ong, Chun Kit Kwok, Hao Sun, Huating Wang DNA G-quadruplex profiling in skeletal muscle stem cells reveals functional and mechanistic insights Genome Biology, 2025, DOI: 10.1186/s13059-025-03753-w
- Jieyu Zhao, Feng Yang, Yuwei Zhang, Huating Wang, Chun Kit Kwok TDP-43 binds to RNA G-quadruplex structure and regulates mRNA stability and translation. Nucleic Acids Res; 2025, DOI: 10.1093/nar/gkaf820
- Kelly Yichen Li, Qin Cao, Savio Ho-Chit Chow, Chiara Nicoletti, Pier Lorenzo Puri, Huating Wang, Danny Leung, Kevin Y. Yip; Regulatory roles of three-dimensional structures of chromatin domains. Genome Biology; 2025, DOI: 10.1186/s13059-025-03659-7
- Yang Li, Chuhan Li, Qin Zhou, Xingyuan Liu, Yulong Qiao, Ting Xie, Hao Sun, Michael Tim-Yun Ong, Huating Wang; Multiomics and cellular senescence profiling of aging human skeletal muscle uncovers Maraviroc as a senotherapeutic approach for sarcopenia. Nature Communications, 2025, DOI: 10.1038/s41467-025-61403-y
- Kun Zhang, Qichang Nie, Maolin Li, Xiaona Chen, Liting Zhong, Tianle Dai, Xiaofan Guo, Haizhou Zhao, Terrence Chi-Kong Lau, Huating Wang, Shuo-Bin Chen, Chun Kit Kwok; RNA G-quadruplex structure-based PROTACs for targeted DHX36 protein degradation and gene activity modulation in mammalian cells. Nucleic Acids Res., 2025, DOI: 10.1093/nar/gkaf039
- Yang Li, Chuhan Li, Qiang Sun, Xingyuan Liu, Fengyuan Chen, Yeelo Cheung, Yu Zhao, Ting Xie, Bénédicte Chazaud, HaoSun, Huating Wang; Skeletal muscle stem cells modulate niche function in Duchenne muscular dystrophy mouse through YY1-CCL5 axis. Nature Communications, 2025, DOI: 10.1038/s41467-025-56474-w
- Ning Huang, Meiling Ge, Xiaolei Liu, Xu Tian, Pengbin Yin, Zhijun Bao, Feng Cao, Ng Shyh-Chang, Biao Dong, Lunzhi Dai, Zhenji Gan, Ping Hu, Jing Qu, Si Wang, Huating Wang, Qian Xiao, Rui Yue, Jirong Yue, Licheng Zhang, Yong Zhang, Hongbo Zhang, Weiqi Zhang, Guang-Hui Liu, Gang Pei, Yong Liu, Dahai Zhu, Birong Dong; A framework of biomarkers for skeletal muscle aging: a consensus statement by the Aging Biomarker Consortium. Life Medicine, lnaf001, 2025, DOI: 10.1093/lifemedi/lnaf001
- Qiang Sun, Qin Zhou, Yulong Qiao, Xiaona Chen, Hao Sun, Huating Wang; Pervasive RNA-binding protein enrichment on TAD boundaries regulates TAD organization. Nucleic Acids Res; 2025, DOI: 10.1093/nar/gkae1271
2024
- Xing Zhao , Zigui Chen, Huating Wang, Hao Sun*; Occlusion enhanced pan-cancer classification via deep learning. BCBioinformatics, 2024, DOI: 10.1186/s12859-024-05870-y
- Yuwei Zhang, Jieyu Zhao, Xiaona Chen, Yulong Qiao, Jinjin Kang, Xiaofan Guo, Feng Yang, Kaixin Lyu, Yiliang Ding, Yu Zhao, Hao Sun, Chun Kit Kwok, Huating Wang; DHX36 binding induces RNA structurome remodeling and regulates RNA abundance via m6A reader YTHDF1. Nature Communications, 2024, DOI: 10.1038/s41467-024-54000-y
- Liqiang He, Hao Sun, Huating Wang; 3D organization of enhancers in MuSCs. Current Topics in Developmental Biology, 2024, DOI: 10.1016/bs.ctdb.2024.01.011
- Chen, X., Y. Li, J. Xu, Y. Cui, Q. Wu, H. Yin, Y. Li, C. Gao, L. Jiang, H. Wang, Z. Wen, Z. Yao, and Z. Wu; Styxl2 regulates de novo sarcomere assembly by binding to non-muscle myosin IIs and promoting their degradation. eLife, 2024, DOI: 10.7554/eLife.87434.2
2023
- Zhiming He, Xiaona Chen, Gexin Liu, Yuying Li, Feng Yang, Hao Sun, Huating Wang; Sugt1 loss in skeletal muscle stem cells impairs muscle regeneration and causes premature muscle aging. Life Medicine, 2023, DOI: 10.1093/lifemedi/lnad039
- Stephanie N. Oprescu, Nick Baumann, Xiyue Chen, Qiang Sun, Yu Zhao, Feng Yue, Huating Wang & Shihuan Kuang; Sox11 is enriched in myogenic progenitors but dispensable for development and regeneration of the skeletal muscle. Skeletal Muscle, 2023, DOI: 10.1186/s13395-023-00324-0
- Suyang Zhang, Feng Yang, Yile Huang, Liangqiang He, Yuying Li, Yi Ching Esther Wan, Yingzhe Ding, Kui Ming Chan, Ting Xie, Hao Sun & Huating Wang; ATF3 induction prevents precocious activation of skeletal muscle stem cell by regulating H2B expression. Nature Communications, 2023, DOI: 10.1038/s41467-023-40465-w
- YU ZHAO, YINGZHE DING, LIANGQIANG HE, QIN ZHOU, XIAONA CHEN, YUYING LI, MARIA VITTORIA ALFONSI, ZHENGUO WU, HAO SUN, HUATING WANG; Multiscale 3D genome reorganization during skeletal muscle stem cell lineage progression and aging. Science Advances, 2023, DOI: 10.1126/sciadv.abo1360
- Yulong Qiao, Qiang Sun, Xiaona Chen, Liangqiang He, Di Wang, Ruibao Su, Yuanchao Xue, Hao Sun, Huating Wang; Nuclear m6A reader YTHDC1 promotes muscle stem cell activation/proliferation by regulating mRNA splicing and nuclear export. eLife, 2023, DOI: 10.7554/eLife.82703
- Liangqiang He, Zhiming He, Yuying Li, Hao Sun, Huating Wang; In Vivo Investigation of Gene Function in Muscle Stem Cells by CRISPR/Cas9-Mediated Genome Editing. Skeletal Muscle Stem Cells, 2023, DOI: 10.1007/978-1-0716-3036-5_21
2022
- Chien Ting Cheng, Dan Wang, Oscar Kuang-Sheng Lee, HUATING WANG, Dai Fei Elmer Ker; Application of neural networks on in vitro-generated Raman spectra for label-free, ex vivo skeletal muscle detection. Measurement, 2022, DOI: 10.1016/j.measurement.2022.112172
- Karl Kam Hei So, Yile Huang, Suyang Zhang, Yulong Qiao, Liangqiang He, Yuying Li, Xiaona Chen, Mai Har Sham, Hao Sun, Huating Wang; seRNA PAM controls skeletal muscle satellite cell proliferation and aging through trans regulation of Timp2 expression synergistically with Ddx5. Aging Cell, 2022, DOI: 10.1111/acel.13673
- Di Liu, Kevin Y. Yang, Vicken W. Chan, Wenchu Ye, Charing C.N. Chong, Chi Chiu Wang, Huating Wang, Bin Zhou, Kenneth K.Y. Cheng, Kathy O. Lui; YY1 Regulates Glucose Homeostasis Through Controlling Insulin Transcription in Pancreatic β-Cells. Diabetes, 2022, DOI: 10.2337/db21-0695
- Xiaona Chen, Guang Xue, Jieyu Zhao, Yuwei Zhang, Suyang Zhang, Wen Wang, Yang Li, Jie Yuan, Liangqiang He, Chun Yin Chan, Yan Liu, Wei Chen, Yu Zhao, Ping Hu, Hao Sun, Chun Kit Kwok, Huating Wang; Lockd promotes myoblast proliferation and muscle regeneration via binding with DHX36 to facilitate 5′ UTR rG4 unwinding and Anp32e translation. Cell Reports, 2022, DOI: 10.1016/j.celrep.2022.110927
- Lifang Han, Gang Wang, Shaopu Zhou, Chenghao Situ, Zhiming He, Yuying Li, Yudan Qiu, Yu Huang, Aimin Xu, Michael Tim Yun Ong, Huating Wang, Jianfa Zhang, Zhenguo Wu; Muscle satellite cells are impaired in type 2 diabetic mice by elevated extracellular adenosine. Cell Reports, 2022, DOI: 10.1016/j.celrep.2022.110884
- Liang C, Ke Q, Liu Z, Ren J, Zhang W, Hu J, Wang Z, Chen H, Xia K, Lai X, Wang Q, Yang K, Li W, Wu Z, Wang C, Yan H, Jiang X, Ji Z, Ma M, Long X, Wang S, Wang H, Sun H, Belmonte JCI, Qu J, Xiang AP, Liu GH; BMAL1 moonlighting as a gatekeeper for LINE1 repression and cellular senescence in primates. Nucleic Acids Res, 2022, DOI: 10.1093/nar/gkac146
2021
- Hongye Wang, Yile Huang, Ming Yu, Yang Yu, Sheng Li, Huating Wang, Hao Sun, Bing Li, Guoliang Xu, Ping Hu; Muscle regeneration controlled by a designated DNA dioxygenase. Cell Death & Disease, 2021, DOI: 10.1038/s41419-021-03817-2
- Liangqiang He, Yingzhe Ding, Yu Zhao, Karl K. So, Xianlu L. Peng, Yuying Li, Jie Yuan, Zhiming He, Xiaona Chen, Hao Sun, Huating Wang; CRISPR/Cas9/AAV9-mediated in vivo editing identifies MYC regulation of 3D genome in skeletal muscle stem cell. Stem Cell Reports, 2021, DOI: 10.1016/j.stemcr.2021.08.011
- Xiaona Chen, Jie Yuan, Guang Xue, Silvia Campanario, Di Wang, Wen Wang, Xi Mou, Shiau Wei Liew, Mubarak Ishaq Umar, Joan Isern, Yu Zhao, Liangqiang He, Yuying Li, Christopher J. Mann, Xiaohua Yu, Lei Wang, Eusebio Perdiguero, Wei Chen, Yuanchao Xue, Yoshikuni Nagamine, Chun Kit Kwok, Hao Sun, Pura Muñoz-Cánoves, Huating Wang; Translational control by DHX36 binding to 5′UTR G-quadruplex is essential for muscle stem-cell regenerative functions. Nature Communications, 2021, DOI: 10.1038/s41467-021-25170-w
- Yile Huang, Yulong Qiao, Yu Zhao, Yuying Li, Jie Yuan, Jiajian Zhou, Hao Sun, Huating Wang; Large scale RNA-binding proteins/LncRNAs interaction analysis to uncover lncRNA nuclear localization mechanisms. Briefings in Bioinformatics, DOI: 10.1093/bib/bbab195
2020
- Kelahmetoglu Y, Jannig PR, Cervenka I, Koch LG, Britton SL, Zhou J, Wang H, Robinson MM, Nair KS, Ruas JL; Comparative Analysis of Skeletal Muscle Transcriptional Signatures Associated With Aerobic Exercise Capacity or Response to Training in Humans and Rats. Front Endocrinol (Lausanne), 10.3389/fendo.2020.591476
- Zhang M, Lai Y, Krupalnik V, Guo P, Guo X, Zhou J, Xu Y, Yu Z, Liu L, Jiang A, Li W, Abdul MM, Ma G, Li N, Fu X, Lv Y, Jiang M, Tariq M, Kanwal S, Liu H, Xu X, Zhang H, Huang Y, Wang L, Chen S, Babarinde IA, Luo Z, Wang D, Zhou T, Ward C, He M, Ibañez DP, Li Y, Zhou J, Yuan J, Feng Y, Arumugam K, Di Vicino U, Bao X, Wu G, Schambach A, Wang H,Sun H, Gao F, Qin B, Hutchins AP, Doble BW, Hartmann C, Cosma MP, Qin Y, Xu GL, Chen R, Volpe G, Chen L, Hanna JH, Esteban MA; β-Catenin safeguards the ground state of mousepluripotency by strengthening the robustness of the transcriptional apparatus. Sci Adv, 10.1126/sciadv.aba1593
- Fan F, Chen D, Zhao Y, Wang H, Sun H, Sun K*; Rapid preliminary purity evaluation of tumor biopsies using deep learning approach. Comput Struct Biotechnol J, 10.1016/j.csbj.2020.06.007
- Kun Sun, Lishi Li, Li Ma, Yu Zhao, Lin Deng, Wang H, Hao Sun. Msuite: A High-Performance and Versatile DNA Methylation Data-Analysis Toolkit. Patterns,2020 https://doi.org/10.1016/j.patter.2020.100127.
- Gene Chi Wai Man, Jianzhang Wang, Yi Song, Jack Ho Wong, Yu Zhao, Tat San Lau, Kam Tong Leung, Tak Hang Chan, Huating Wang, Joseph Kwong, Tzi Bun Ng & Chi Chiu Wang; Therapeutic potential of a novel prodrug of green tea extract in induction of apoptosis via ERK/JNK and Akt signaling pathway in human endometrial cancer. BMC Cancer, https://doi.org/10.1093/bib/bbab195
- Sun, K., Wang H & Sun, H; NAMS webserver: coding potential assessment and functional annotation of plant transcripts. Briefings in Bioinformatics, https://doi.org/10.1093/bib/bbaa200
- Shen’ao Zhou, Wei Zhang, Gaihong Cai, Yingzhe Ding, Caixia Wei, Sheng Li, Yu Yang, Jie Qin, Dan Liu, Hao Zhang, Xiexiang Shao, Jianhua Wang, Hongye Wang, Wenjun Yang, Huating Wang, She Chen, Ping Hu & Liming Sun; Myofiber necroptosis promotes muscle stem cell proliferation via releasing Tenascin-C during regeneration. Cell Research, https://doi.org/10.1038/s41422-020-00393-6
- Yuying Li, Jie Yuan, Fengyuan Chen, Suyang Zhang, Yu Zhao, Xiaona Chen, Leina Lu, Liang Zhou, Ching Yan Chu, Hao Sun, Huating Wang; Long noncoding RNA SAM promotes myoblast proliferation through stabilizing Sugt1 and facilitating kinetochore assembly. Nat Commun, https://doi.org/10.1038/s41467-020-16553-6
- Linlin Hou, Yuanjie Wei, Yingying Lin, Xiwei Wang, Yiwei Lai, Menghui Yin, Yanpu Chen, Xiangpeng Guo, Senbin Wu, Yindi Zhu, Jie Yuan, Muqddas Tariq, Na Li, Hao Sun, Huating Wang, Xiaofei Zhang, Jiekai Chen, Xichen Bao, Ralf Jauch; Concurrent binding to DNA and RNA facilitates the pluripotency reprogramming activity of Sox2. Nucleic Acids Research, https://doi.org/10.1093/nar/gkaa067
2019
2018
2017
- Zhang J, Jiang Y, Zhao Y, Wang W, Xie Y, Wang H, Yang Y. Downregulation of tyrosine threonine kinase inhibits tumor growth via G2/M arrest in human endometrioid endometrial adenocarcinoma. Tumour Biol. 2017 Jul;39(7):1010428317712444.
- Sun K, Wang H, Sun H. mTFkb: a knowledgebase for fundamental annotation of mouse transcription factors. Sci Rep. 2017 Jun 8; 7(1):3022.
- An Y, Wang G, Diao Y, Long Y, Fu X, Weng M, Zhou L, Sun K, Cheung TH, Ip NY, Sun H, Wang H, Wu Z. A Molecular Switch Regulating Cell Fate Choice between Muscle Progenitor Cells and Brown Adipocytes. Dev Cell. 2017 May 22;41(4):382-391.e5.
- Peng X, So KK, He L, Zhao Y, Zhou J, Li Y, Yao M, Xu B, Zhang S, Yao H, Hu P, Sun H, Wang H. MyoD- and FoxO3-mediated hotspot interaction orchestrates super-enhancer activity during myogenic differentiation. Nucleic Acids Res. 2017 Sep 6;45(15):8785-8805.
- Zhou J, Zhang S, Wang H, Sun H. LncFunNet: an integrated computational framework for identification of functional long noncoding RNAs in mouse skeletal muscle cells. Nucleic Acid Res. 2017 Jul 7;45(12):e108.
- Chen X, He L, Zhao Y, Li Y, Zhang S, Sun K, So K, Chen F, Zhou L, Lu L, Wang L, Zhu X, Bao X, Esteban MA, Nakagawa S, Prasanth KV, Wu Z, Sun H, Wang H. Malat1 regulates myogenic differentiation and muscle regeneration through modulating MyoD transcriptional activity. Cell Discov. 2017 Mar 14;3:17002.
- Peng X, Sun K, Zhou J, Sun H, Wang H. Bioinformatics for Novel Long Intergenic Noncoding RNA (lincRNA) Identification in Skeletal Muscle Cells. Peng X, Sun K, Zhou J, Sun H, Wang H. Methods Mol Biol. 2017;1556:355-362.